The Utility of SUDOSCAN® in Asymptomatic Hepatitis C

Detection of Small Fiber Neuropathy in Asymptomatic Hepatitis C Virus-Related Cirrhosis Patients Using SUDOSCAN® Technology
ES. Tharwa et al.
Article title: Sudomotor Changes in Hepatitis C Virus Infection with or without Diabetes Mellitus: A Pilot Study in Egyptian Patients.
Am. J. Trop. Med. Hyg., 00(0), 2020, pp. 1–5.
Tharwa et al from Menoufia University, Egypt, conducted a study to evaluate small fiber neuropathy in asymptomatic Hepatitis C virus-related cirrhotic patients with (group 1, n=49) or without diabetes mellitus (group 2, n=48) using the SUDOSCAN test for sweat function assessment.
Peripheral neuropathy was detected in none of the healthy controls, 39% of Hepatitis C virus-related cirrhotic patients without diabetes mellitus, and 62% of Hepatitis C virus -related cirrhotic patients with type 2 diabetes mellitus (P< 0.0001).
Feet electrochemical skin conductance was decreased in group 1 as compared with controls (67 ± 19 versus 88 ± 7 μS, P < 0.0001) and more severely decreased in group 2 (58 ± 19 μS, P = 0.017 as compared with group 1), see Figure 1.
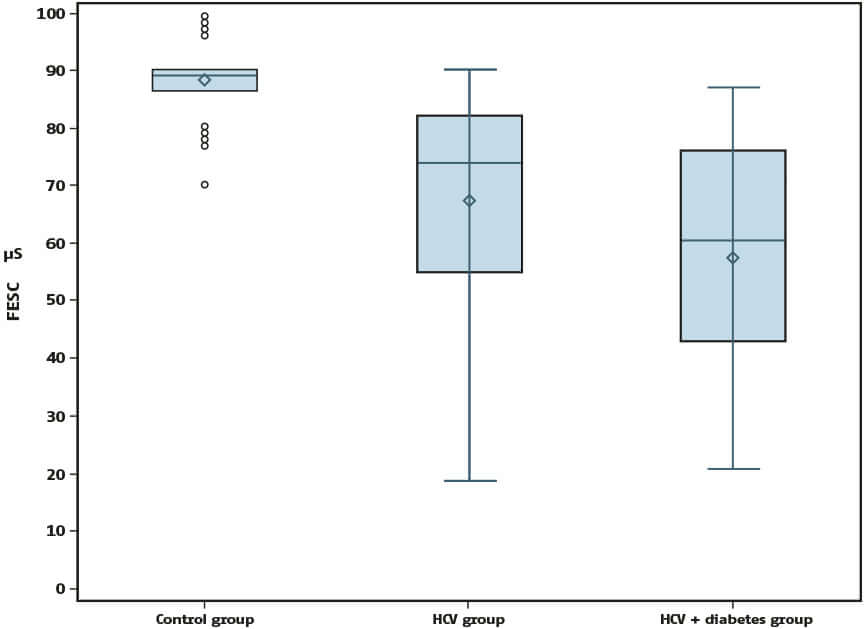
Figure 1: Box-plots of feet electrochemical skin conductance (FESC) in controls, patients with hepatitis C virus (HCV)–related cirrhosis, and patients with HCV-related cirrhosis as well as diabetes mellitus (DM).
Feet electrochemical skin conductance is decreased in HCV-related cirrhosis as compared with controls, and more profoundly decreased in patients with DM in addition.
Moreover, a significant correlation was observed between feet Electrochemical skin conductance measured with SUDOSCAN and parameters of increased portal pressure and parameters of increased severity of hepatic dysfunction, see Table 1.
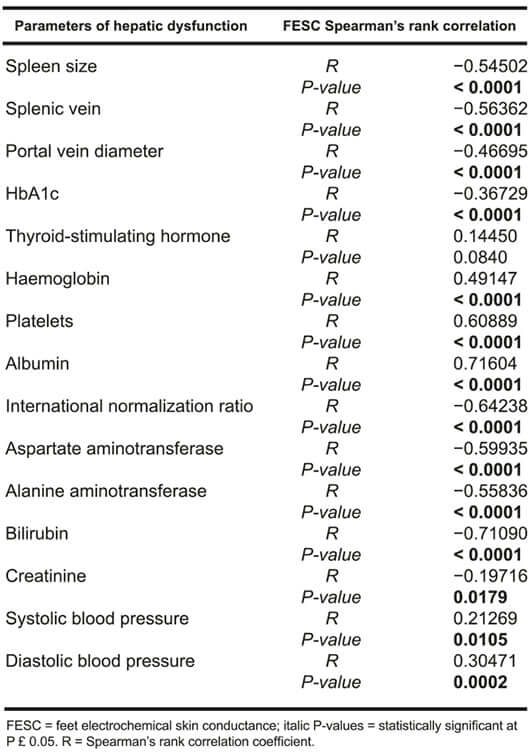
Table 1: Spearman’s correlations between FESC and parameters of increased portal pressure and severity
of hepatic dysfunction
Conclusion: Electrochemical skin conductance measurement is a valuable, noninvasive method for early detection of small fiber neuropathy in asymptomatic Hepatitis C virus-related cirrhosis, with or without diabetes mellitus.



